Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Review on Current Applications and Future Directions in Carbon Nanotubes for Cancer Therapy using AI
Authors: Promod Kumar B M, M M Aatifulla Baig, Shivarudrayya I Dharawadmath, Vikas C M
DOI Link: https://doi.org/10.22214/ijraset.2023.55787
Certificate: View Certificate
Abstract
Carbon nanotubes (CNTs), which are measured in nanometers, provide an advantage over standard pharmaceuticals. As a result of this capability, they may be able to deliver smaller amounts of medication directly to the damaged cells in the body. CNTs accomplish this by lowering the probability of adverse reactions, minimizing injury to healthy cells, and boosting the targeting effectiveness for diseased cells at the same time. CNTs are effective nanocarriers for protein, DNA, and anticancer medicines during chemotherapy. This article covers the structure of carbon nanotubes and emphasizes its use in nano biosensors, cancer imaging, and drug delivery systems.
Introduction
I. INTRODUCTION
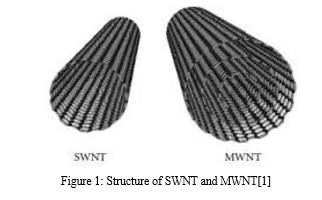
Nanomedicine provides a considerable advantage over traditional therapeutic choices due to its increased passive targeting abilities through higher permeability and retention (EPR), more surface area for drug loading and customization, and prolonged plasma half-life[1]. Carbon nanotubes (CNTs) have drawn the interest of multiple researchers for both cancer treatment and cancer detection due to their versatility in application across various nanocarriers[2]. CNTs are seamless rolls of graphene with a high aspect ratio, a diameter of 1 nm, and a length of many micrometers. They could have a closed end or an open end. SWNTs are formed from a single graphene sheet, as opposed to MWNTs, which are made from several graphene sheets. A number of medicinal drugs can mix or adsorb with them.
The use of CNTs in tissue engineering and brain research is becoming more significant in terms of their biomedical applications. Furthermore, the utilization of CNTs as cutting-edge platforms for the incredibly accurate identification of antibodies linked to autoimmune disorders in people has been demonstrated. The invention of unique complementary DNA strand hybridization methods has enabled ultrasensitive DNA detection. DNA and PNA (peptide nucleic acid), which have been covalently modified on the open ends of carbon nanotubes, are used in these methods[4].
II. CNT IN DRUG DELIVERY SYSTEM(DDS)
Important feature of CNTs is their ability to penetrate cellular barriers and deliver drugs directly to target tissues. Their nanoscale dimensions allow for effective bypassing of biological barriers, such as cell membranes and the blood-brain barrier, facilitating drug delivery to specific sites within the body. This method of focused drug administration reduces side effects and increases therapeutic effectiveness[5].
A. Cellular Uptake of CNTs
Due to their needle-like structure, CNTs might be able to penetrate cellular membranes and reach cellular constituents without obviously harming the cell. As soon as the MWNTs nanoinjector entered the cell and severed the disulfide bond, the payload chemical was discharged into the cytoplasm. The orientation of the nanotubes perpendicular to the cell membranes demonstrates that CNT absorption was equivalent to nanoneedle absorption since nanoneedles can pass through cell membranes without hurting the cells. Even though it was found that shorter nanotubes were simpler to absorb into cells and that endocytosis was not the process of cellular absorption[6, 7], the MWNT uptake methodology is highly dependent on nanotube length.
B. Tumour Targeting
Tumour targeting is a critical aspect of cancer treatment, aiming to deliver therapeutic agents specifically to cancerous cells while minimising damage to healthy tissues. Numerous characteristics of CNTs make them interesting candidates for tumor targeting. Their high surface area-to-volume ratio, first and foremost, enables efficient loading of anti-cancer medicines, such as chemotherapeutic agents, small compounds, or nucleic acids[7]
- Nucleus Targeting
Because it regulates cell proliferation and metabolism, as well as cell cycle control and gene activation, the nucleus is viewed as an appealing therapeutic target for the treatment of malignant tumors. The use of nanoparticles that penetrate the nucleus membrane or target the nucleus will result in more effective cancer treatment. Oh et al. built a DDS for combined NIR-irradiated PTT and chemotherapy using anthracycline, doxorubicin, and PEGylated SWNTs[8]. DOX, a topoisomerase inhibitor, is a frequent drug. The capacity of PEGSWNTs-DOX to concentrate at high concentrations inside cancer cells and successfully move to their nucleus revealed the remarkable potential of PEG-SWNTs-DOX for the treatment of breast cancer[9]. In vitro experiments that confirmed the viability of transporting the complex into MCF-7(Michigan Cancer Foundation) breast cancer cells demonstrates that the f-SWNTs-DNA(functionalized-SWNTs-DNA) complex with p53(protein 53) plasmid could enter the cytoplasm of cancer cells via endocytosis and then reach their nucleus, where the p53 plasmid could be separated from the f-SWNTs vector due to the pH change between the nucleus and cytoplasm. The p53 gene's transcription and translation activated apoptotic pathways in the cytoplasm. f-SWNTs have been shown in previous studies to be potential gene vectors capable of transporting the target gene into the nucleus[10].
2. Cytoplasm Targeting
Because of the complexity of its constituent parts, which comprise various macromolecules, organelles, cytoskeletal networks, and cytosol, the cytoplasm has a highly organized and diversified architecture[11].The cytoplasm, the primary center of cellular activity, is necessary for various basic cellular activities such as cell division and polarization[12]. Because of their capacity to efficiently penetrate cells, CNTs can be used as effective vectors for gene therapies[13].Gene therapy can also be very effective by using siRNA to block the expression of harmful genes. SiRNA is a double-stranded RNA molecule that binds to single-standard mRNA and corresponding double-stranded RNA to impede mRNA translation. This is referred to as mRNA silencing. Chen et al. developed a carbon nanotube-based nano DDS that delivered the chemotherapeutic medicine 5-FU and successfully cured treatment-resistant gastric cancer with peritoneal metastases. The siRNA combination may precisely attach to cancer cells, allowing for full DDS active targeting potential for accurate 5-FU administration. This novel DDS was able to cause 5-FU-resistant gastric cancer cells to die while also restricting their penetration and proliferation in in vitro studies utilizing the gastric cancer cell line MKN45, enhancing the efficacy of chemotherapy[14]. Therapeutic siRNA effectively silenced the drug-resistant gene, demonstrating the potential of this CNT-based nanocarrier as a siRNA and chemotherapeutic delivery method. Therapeutic siRNA significantly inhibited the drug-resistant gene, revealing the superiority of this CNT-based nanocarrier as a siRNA and chemotherapeutic delivery system[15].DWNTs were able to enter mammalian cells due to their nanoneedle shape. They could also escape from lysosomes and release siRNA into the cytoplasm, which killed cancer cells swiftly. Finally, CNT-based nanodevices that transport siRNA to the cytoplasm enable efficient gene therapy[16].
3. Mitochondria Targeting
The mitochondria are one of the most significant organelles in cells. Their length varies and their diameter is approximately one meter. The role of mitochondria in energy metabolism, particularly ATP generation, is well understood[17]. Because mitochondria are the principal source of energy for rapidly multiplying cancer cells, they are an important therapeutic target for the disease[18]. Numerous cancer cells can take up CNTs and can be targeted by mitochondriotropics and other drugs that target mitochondria[17].CNTs can also be used in PTT, photoacoustic therapy, and thermoacoustic therapy (TAT) because of their exceptional optical capabilities to kill cancer cells by directly harming their mitochondria[19]
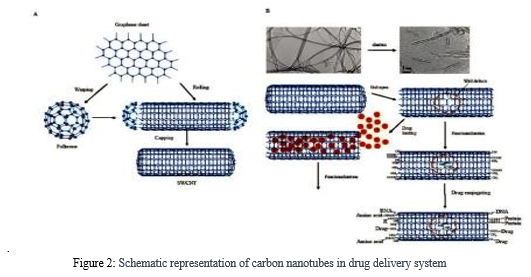 .
.
Figure 2: Schematic representation of carbon nanotubes in drug delivery system
III. CNTS IN CANCER IMAGING
The primary purpose of cancer imaging is to detect and precisely locate malignant cells or tumors within the body. Cancer diagnosis has traditionally depended mainly on conventional imaging procedures such as X-rays, MRIs, and computed tomography (CT) scans. They typically have drawbacks such as poor resolution, low sensitivity, and the potential to harm healthy cells[20]. Carbon nanotubes (CNTs) are increasingly being used in cancer imaging, revealing a key scientific and therapeutic perspective on cancer diagnosis. Raman imaging, nuclear magnetic resonance imaging (NMR), ultrasonography, photoacoustic imaging, and fluorescence imaging are some of the imaging techniques used for this[21].
A. Raman Imaging
The Raman scattering technique is used to excite light and convey photon emission wavelengths. The tangent Gmodule (TGM) and the radial breathing model[22] are two examples of many high Raman peaks that CNTs display as a result of the acute electronic density of states near the van Hove singularities (RBM).Raman microscopy was utilized to examine the dispersion of o-SWNTs-PEG in colon-26 cells. Sekiyama et al. produced epoxide type oxygen-doped SWNTs with PEG modification (o-SWNTs- PEG), a recently generated over-thousand-manometer (OTN)-NIR fluorescent probe, to study the time-dependent fluctuation in OTN-NIR fluorescence pictures of colon-26 cancer cells[23]. According to the experiment results, the Raman signals recorded from this generated nanostructure in colon-26 cells were much higher on the fifth day.
B. Radiography Using Nuclear Magnetic Resonance
When employing MRI, a non-invasive imaging method, the human body is not subjected to ionizing radiation. It can provide accurate 3D cross-section pictures and has considerable medical imaging benefits[25].CNTs are extensively employed in therapeutic settings as contrast agents to improve nuclear magnetic resonance (MR) imaging. MRI scanners are commonly used to create these MR images[26]. Yan et al. employed carbon nanotubes to non-covalently combine the NGR (asparagine-glycine-arginine) peptide, DOX, and a type of magnetic resonance contrast agent, Gd DTPA. Because of its extraordinary anticancer efficacy and MRI capabilities, this nanotechnology has a surprising synergistic effect on tumor detection and treatment[27].
C. Ultrasonography
Due to its low detection cost and inherent safety, ultrasound is a fundamental and well-known diagnostic imaging technology[28].The outcomes of the ultrasonic imaging revealed that these NPs displayed notable contrast at each dose tested, suggesting that they might develop into a specific kind of contrast agent for ultrasonography. The application of MWNTs in ultrasonography was studied by Delogu et al. [29].
D. Photoacoustic Imaging
Photoacoustic imaging (PAI) is a unique imaging technology that has lately acquired prominence in a variety of biological applications[30].PAI outperforms existing optical imaging technologies in terms of spatial resolution and the ability to view deeper tissues[31].MWNTs and SWNTs were employed as photothermal agents due to their significant NIR (near infrared radiation) absorption[32]. When compared to controls, SWNTs can increase the signal in thermoacoustic tomography (TAT) and photoacoustic tomography (PAT) by more than two and six times, respectively. Because SWNTs provide the most signal when compared to other carbon materials, fullerenes, and graphitic microparticles[33], they may be used as suitable contrast-mediums in the PAI.Gold-coated layers or bioorganic compounds must be introduced to boost the absorbance of SWNTs in the NIR range. Some researchers have developed "golden nanotubes" (GNTs) with a gold coating to increase the photoacoustic signal emitted by SWNTs[34].
E. Fluorescence Imaging
Fluorescence imaging (FI) plays critical functions in the diagnosis of medical disorders. However, due to their restricted penetration depth, their utility in fluorescence imaging has been limited[35].To address this issue, some scientists have been working tirelessly to develop and improve fluorescence probes.
These probes' wavelength range can be triggered and sent to the physiologically transparent NIR window[36].Robinson and his associates.
A SWNTs formulation with a 30 hour in vivo half-life and an accumulation of more than 30% of the drug's inject dose (percent ID/g) was developed[37].For the first time, fluorescent signals in cancers and other organs were detected using high fluorescence video rate imaging and principal component analysis (PCA). After a procedure, they could clearly observe fluorescent signals inside the tumor. IV. CNTs IN NANOBIOSENSORS
Nano biosensors have gained popularity in recent years, owing to their ease of use, speed, and affordability[38].Because of their small size, certain biological substances can be identified and analyzed with greater sensitivity and specificity. Nano biosensors typically combine biological recognition components with nanomaterials with sizes between 1 and 100 nm[39].CNTs are employed as biosensors because of their exceptional properties, including enhanced electrocatalytic activity, excellent stability with delayed oxidation kinetics, and better electrical conductivity[40].
A. Combination of CNT with Antibodies
A nano immunosensor is a form of nano biosensor that detects cancer by using antibodies. The osteopathic (OPN) antibody can identify prostate cancer [41].
An ELISA test is often used to evaluate OPN despite its low sensitivity, high cost, and complicated operation.Sharma et al. demonstrate that by incorporating OPN monoclonal antibodies on the surface of Carboxylated SWNTs, they may be able to develop a nano immunosensor for the electrical detection of prostate cancer cells[43].
B. Combination of CNT with Metallic Compounds
To make nano biosensors, carbon nanotubes can be combined with metallic nanoparticles such as AuNPs and AgNPs. MiR-21, a form of micro ribonucleic acid, was electrochemically detected by MWNTs modified with AuNPs, according to research[44].MiR-21, derived from body fluids, aids in the early diagnosis of pancreatic cancer. However, miR-21 levels in the blood are usually low in the early stages of pancreatic cancer, making cancer identification extremely difficult. The addition of AuNPs-decorated MWNTs enhanced the detection of miR-21 significantly[45].

Conclusion
Carbon nanotubes (CNTs) have sparked a lot of interest in the biological sectors due to their unique topologies and features, including as high aspect ratios, large surface areas, a wide range of surface chemical activities, and nanoscale size stability. Anticancer drugs such as DOX, CPT, CP, CDDP, PTX, and DTX are delivered using CNT as a nanocarrier. Despite multiple research demonstrating promising in vitro and in vivo results for CNT-based therapies, there are several limits to their therapeutic use. For starters, long-term safety problems in the human body have received little attention. CNTs have been surface-functionalized and purified to reduce toxicity. To increase the activity and stability of drug-CNTs, a range of CNT functionalization techniques involving diverse chemicals and materials have been reported. As more research enhances these approaches for use in real-world applications, carbon nanotubes will rank among the most successful instruments in a range of biological areas, including cancer therapy.
References
[1] L. Tang, Q. Xiao, Y. Mei, S. He, Z. Zhang, R. Wang, and W. Wang. Perspectives on the application of functionalized carbon nanotubes in cancer theranostics. Vol. 19, No. 1 of the Journal of Nanobiotechnology. The company BioMed Central, Inc. doi:10.1186/s12951-021-01174-y [2] Rao, R., Pint, C. L., Islam, A. E., Weatherup, R. S., Hofmann, S., Meshot, E. R., Wu, F., Zhou, C., Dee, N., Amama, P. B., Carpena-Nuez, J., Shi, W. Carbon Nanotubes and Related Nanomaterials: Advances and Challenges in Synthesis. The findings were published in the journal ACS Nano (Vol. 12, Issue 12, pp. 11756-11784). The American Chemical Society is a professional scientific society. https://doi.org/10.1021/acsnano.8b06511 [3] A. Bianco, K. Kostarelos, C. D. Partidos, and A. Prato M. (2005). Carbon nanotubes with functionalized surfaces have biomedical applications. Chemical Communications, Volume 5, Issue 5, pp. 571-577. The Royal Society of Chemistry is a scientific organization. https://doi.org/10.1039/b410943k [4] Among others who have contributed to this work are D. Pantarotto, L. Lacerda, G. Pastorin, C. dric Klumpp, M. Prato, A. Bianco, and K. Kostarelos. Carbon nanotube radiotracer tissue biodistribution and blood clearance rates were studied after intravenous administration. www.pnas.orgcgidoi10.1073pnas.0509009103 [5] H. He, C. Pham-Huy, L. A. Pham-Huy, P. Dramou, D. Xiao, P. Zuo, and C. Pham-Huy. Carbon nanotubes are used in pharmaceutical and medical applications. 2013; BioMed Research International. https://doi.org/10.1155/2013/578290 [6] A team of researchers led by Kostalos K., Lacerda L., Pastorin G., Wu W., Wieckowski S., Luangsivilay J., Godefroy S., Pantarotto D., Briand J. P., Muller S., and Bianco A. Regardless of the functional group or kind of cell, cells can absorb functionalized carbon nanotubes. Nature Nanotechnology, volume 2, issue 2, DOI: 10.1038/nnano.2006.209 108-113 [7] S. S. Bhattacharya, A. K. Mishra, N. Verma, A. Verma, and J. K. Pandit (2014). Journal of Drug Delivery, 2014. Carbon Nanotubes: An Emerging Drug Carrier for Cancer Cell Targeting, 1-23. https://doi.org/10.1155/2014/670815 [8] Oh, Y., Jin, J. O., and Oh, J. For the successful death of human breast cancer cells, photothermal-triggered regulation of subcellular drug accumulation utilizing doxorubicin-loaded single-walled carbon nanotubes was used. 28(12), nanotechnology. DOI: 10.1088/1361-6528/aa5d7d [9] M. J. Shieh, P. C. Lee, Y. C. Chiou, J. M. Wong, C. L. Peng, and P. C. Lee (2013). Single-walled carbon nanotubes combined with the anticancer medication SN 38 and an EGFR antibody are being used to target colorectal cancer cells. 87568765 in Biomaterials, vol. 34(34). https://doi.org/10.1016/j.biomaterials.2013.07.067 [10] S. E. Kern, K. W. Kinzler, Bruskin, Jarosz, Friedman, Prives, and Vogelstein 1989. p53 has been identified as a sequencespecific DNA-binding protein. The Journal of Cell Physiology has published its 28th volume. www.sciencemag.org [11] S. Etienne-Manneville (2018). In Cell Biology, Cytoplasmic Intermediate Filaments. Annu. Rev. Cell Dev. Biol., 34, 33. Annu. Rev. Cell Dev. Biol., 34, 33. https://doi.org/10.1146/annurev-cellbio-100617 [12] S. Shimapour, S. Caballero-Mancebo, and C. P. Heisenberg (2021). Cytoplasm is in motion. pp. 213-226 in Developmental Cell, vol. 56, no. 2. Cell Press. https://doi.org/10.1016/j.devcel.2020.12.002 [13] A. Singh, M. Hua Hsu, N. Gupta, P. Khanra, P. Kumar, V. Prakash Verma, and M. Kapoor. Derivatized Carbon Nanotubes for Gene Therapy in Mammalian and Plant Cells. Vol. 85, No. 3, pp. 466-475 in ChemPlusChem. Wiley-VCH Verlag is a publishing house. https://doi.org/10.1002/cplu.201900678 [14] SiRNA delivery system methods, design, and chemistry. Dong, Y., Siegwart, D. J., and Anderson, D. G. Pages 133-147 in Advanced Drug Delivery Reviews, Volume 144. The company Elsevier B.V. doi:10.1016/j.addr.2019.05.004 15 [15] J. M. Lee, T. J. Yoon, and Y. S. Cho. Recent breakthroughs in siRNA delivery using nanoparticles for cancer treatment. BioMed Research International (2013, Vol. doi:10.1155/2013/782041 [16] M. A. Herrero, F. M. Toma, K. T. Al-Jamal, K. Kostarelos, A. Bianco, T. da Ros, F. Bano, L. Casalis, G. Scoles, and M. Prato. For successful siRNA delivery, a carbon nanotube-dendron series was produced and described. Journal of the American Chemical Society, 131(28), pp. 9843-9848. https://doi.org/10.1021/ja903316z [17] P. Maher, A. Currais, D. Soriano-Castell, Z. Liang, and A. Currais (2021). Emerging therapeutics for age-related neurological illnesses include natural chemicals that target mitochondria. 221st volume of Pharmacology and Therapeutics. Elsevier, Inc. https://doi.org/10.1016/j.pharmthera.2020.107749 [18] Mani, S., Swargiary, G., and Singh, K. K. (2020). Natural medicines that target mitochondria are utilized to treat cancer. This study was published in the International Journal of Molecular Sciences (Vol. 21, Issue 19, pp. 1-30). MDPI AG. https://doi.org/10.3390/ijms21196992 [19] B. Kang, D. Yu, Y. Dai, S. Chang, D. Chen, and Y. Ding. Carbon nanotubes as \\\"bomb\\\" agents for cancer cell targeting and photoacoustic therapy. Small, 5(11), 1292-1301. https://doi.org/10.1002/smll.200801820 [20] J. Xu, J. Long, C. Jin, D. liang Fu, Q. xing Ni, and X. jun Yu. Carbon nanotubes are being used in cancer diagnosis and treatment. Cancer Reviews (Vol. 1806, Issue 1, pp. 29-35), Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbcan.2010.02.004 [21] L. Biasutto, M. Zoratti, and I. Szabo (2021). Cancer treatment using mitochondrial ion channels. Redox Biology 42. https://doi.org/10.1016/j.redox.2020.101846 [22] Z. Liu, S. Tabakman, K. Welsher, and H. Dai. In biology and medicine, carbon nanotubes are used for detection, imaging, and medication administration in vitro and in vivo. Nano Research, 2(2), pp. 85-120. https://doi.org/10.1007/s12274-009-9009-8 [23] S. Sekiyama, M. Umezawa, Y. Iizumi, T. Ube, T. Okazaki, M. Kamimura, and K. Soga (2019). Murine Cancer Cells Labeled with Oxygen-Doped Single-Walled Carbon Nanotubes Show a Delayed Increase in Near-Infrared Fluorescence. 831-837 in Langmuir 35(3). https://doi.org/10.1021/acs.langmuir.8b03789 [24] SERS substrates are silver-decorated carbon nanotube networks, as detailed in Journal of Raman Spectroscopy, 1255-1262 by Chen, Y. C., Young, R. J., MacPherson, J. v., and Wilson, N. R. https://doi.org/10.1002/jrs.2862 [25] (2019) J. Su, K. Wu, W. Ma, B. Wang, M. Li, P. Sun, Q. Shen, Q. Wang, and Q. Fan. Natural Phase-Change MaterialBased Multifunctional Thermosensitive Liposomes: Drug Release Triggered by Near-Infrared Light and Multimodal ImagingGuided Cancer Combination Therapy. Interfaces and Applied Materials, 10540-10553. https://doi.org/10.1021/acsami.8b22748 [26] Ghaghada, K. B., Starosolski, Z. A., Bhayana, S., Stupin, I., Patel, C. v., Bhavane, R. C., Gao, H., Bednov, A., Yallampalli, C., Belfort, M. A nanoparticle-based blood-pool contrast agent for placental MR imaging is being tested in pre-clinical trials. 60-70 Placenta is 57 years old. https://doi.org/10.1016/j.placenta.2017.06.008 [27] (2017). Yan, C., Chen, C., Hou, L., Zhang, H., Che, Y., Qi, Y., Zhang, X., Cheng, J., and Zhang, Z. Doxorubicin and GdDTPA loaded single-walled carbon nanotubes for targeted drug administration and magnetic resonance imaging. Drug Targeting, 25(2), pp. 163-171. https://doi.org/10.1080/1061186X.2016.1221958 [28] A. Sanginario, B. Miccoli, and D. Demarchi (2017). Carbon Nanotubes as a Potential Cancer Diagnosis and Treatment. Biosensors (Vol. 7, No. 1). MDPI. doi:10.3390/bios7010009. [29] Nicolussi, P., Ligios, C., Bedognetti, D., Sgarrella, F., Manetti, R., Delogu, L. G., Vidili, G., Venturelli, E., MénardMoyon, C., Zoroddu, M. A., Pilo, G., Manetti, R. Functionalized multiwalled carbon nanotubes were employed as ultrasonic contrast agents. Proceedings of the National Academy of Sciences of the United States of America, 109(41), 16612-16617. https://doi.org/10.1073/pnas.1208312109 [30] J. Simon, E. Flahaut, and M. Golzio (2019). Carbon nanotubes in biomedical applications: an overview. Materials (Vol. 12, No. 4). MDPI AG. https://doi.org/10.3390/ma12040624 [31] Yang, K., Hu, L., Ma, X., Ye, S., Cheng, L., Shi, X., Li, C., Li, Y., and Liu, Z. Photothermal therapy guided by multimodal imaging employing functionalized graphene nanosheets anchored with magnetic nanoparticles. Advanced Materials, vol. 24, no. 14, pp. 1868-1872. https://doi.org/10.1002/adma.201104964 [32] Robinson, J. T., Welsher, S. M. Tabakman, Sherlock, S. P., H. Wang, R. Luong, and Dai, H. 779-793 in Nano Research, 3(11). Carbon nanotubes provide high-performance in vivo near-IR (>1 m) imaging and photothermal cancer therapy. https://doi.org/10.1007/s12274-010-0045-1 [33] M. Pranik, M. Swierczewska, D. Green, B. Sitharaman, and L. v. Wang are the authors of this paper. Carbon nanotubes with single walls as a thermoacoustic and photoacoustic contrast agent in many modes. Journal of Biomedical Optics, 14(3), 034018. https://doi.org/10.1117/1.3147407 [34] E. I. Galanzha, J. W. Kim, E. v. Shashkov, H. M. Moon, and V. P. Zharov. Golden carbon nanotubes operate as photoacoustic and photothermal high-contrast molecular agents in a variety of modes. Nature Nanotechnology, vol. 4, no. 10, pp. 688-694. https://doi.org/10.1038/nnano.2009.231 [35] .Bu, L., Shen, B., and Z. Cheng (2014). Fluorescent imaging of malignant tissues in preparation for targeted surgery. Advanced Drug Delivery Reviews, Vol. 76, No. 1, pp. 21-38. Elsevier. https://doi.org/10.1016/j.addr.2014.07.008 [36] Li, C., Chen, G., Zhang, Y., Wu, F., and Wang, Q. Advanced fluorescence imaging technology for biomedical applications in the near-infrared-II window. American Chemical Society Journal (Vol. 142, Issue 35, pp. 14789-14804). The American Chemical Society is a scientific organization. https://doi.org/10.1021/jacs.0c07022 [37] J. T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi, and H. Dai (2012). Long circulating carbon nanotubes capable of ultrahigh tumor uptake were used for in vivo fluorescence imaging in the second near-infrared window. American Chemical Society Journal, 134(25), pp. 10664-10669. https://doi.org/10.1021/ja303737a [38] S. Gupta, C. N. Murthy, and C. R. Prabha (2018). Recent developments in electrochemical biosensors based on carbon nanotubes. The International Journal of Biological Macromolecules (Vol. 108, pp. 687–703) published the findings. Elsevier B.V., doi:10.1016/j.ijbiomac.2017.12.038 [39] D. Moon, Y. K. Cha, S. ong Kim, S. Cho, H. J. Ko, and T. H. Park (2020). Nanobiosensors based on FETs for smell and taste sensing. Science China Life Sciences (Vol. 63, No. 8), pp. 1159-1167. Press for Science in China. https://doi.org/10.1007/s11427-019-1571-8 [40] M. Yoosefian and N. Etminan (2018). A new nanobiosensor for protein detection in silicon is a leucine/Pd-loaded (5,5) single-walled carbon nanotube matrix. Amino Acids, vol. 50, no. 6, pp. 653-661. https://doi.org/10.1007/s00726-018-2552-4 [41] Popovics, P., Awadallah, W. N., Kohrt, S. E., Case, T. C., Miller, N. L., Ricke, E. A., Huang, W., Ramirez-Solano, M., Liu, Q., Vezina, C. M., Matusik, R. J. Symptomatic benign prostatic hyperplasia is associated with prostatic osteopontin expression. 731-741 in Prostate, 80(10). https://doi.org/10.1002/pros.23986 [42] Li X., Zhang Y.-P., Kim H.-S., Bae K.-H., Stantz K. M., Lee S.-J., Jung C., Jiménez J. A., Gardner T. A., Jeng M.-H., and Kao C. PSES Enhancer Controls Adenovirus E1a and E4 Gene Expression in Prostate Cancer Patients. www.aacrjournals.org [43] A. Sharma, S. Hong, R. Singh, and J. Jang (2015). Transparent immunosensor based on single-walled carbon nanotubes for detection of the prostate cancer biomarker osteopontin. Analytica Chimica Acta, vol. 869, pp. 68-73. https://doi.org/10.1016/j.aca.2015.02.010 [44] H. A. Rafiee-Pour, M. Behpour, and M. Keshavarz (2016). A novel label-free electrochemical miRNA biosensor based on methylene blue as a redox indicator, with application to the breast cancer biomarker miRNA-21. 202-207 in Biosensors and Bioelectronics. https://doi.org/10.1016/j.bios.2015.09.025 [45] T. Kilic, A. Erdem, M. Ozsoz, and S. Carrara. MicroRNA biosensors: Opportunities and challenges in conventional and commercially available methods. Pages 525-546 in Biosensors and Bioelectronics, Volume 99. The Elsevier Inc. https://doi.org/10.1016/j.bios.2017.08.007 [46] Kostarelos, K., Bianco, A., and Prato, M. Carbon nanotube imaging and therapy promises, facts, and constraints. Nature Nanotechnology, 4(10), pp. 627-633. https://doi.org/10.1038/nnano.2009.241 [47] A. Bianco, K. Kostarelos, and M. Prato (2005). Carbon nanotubes have use in medication delivery. Current Opinion in Chemical Biology, Vol. 9, No. 6, pp. 674-679. https://doi.org/10.1016/j.cbpa.2005.10.005 [48] M. Zhang, W. Wang, Y. Cui, N. Zhou, and J. Shen (2018). Multiwalled Carbon Nanotubes with Magnetofluorescent Carbon Quantum Dots for Dual-Modal Targeted Imaging in Chemo-Photothermal Synergistic Therapy. 4(1), 151-162 in ACS Biomaterials Science and Engineering. https://doi.org/10.1021/acsbiomaterials.7b00531 [49] D. Hiraoka, E. Hosoda, K. Chiba, and T. Kishimoto (2019). At the meiotic G2/M transition, SGK phosphorylates Cdc25 and Myt1 to activate cyclin B-Cdk1. 3597-3611 in Journal of Cell Biology, 218(11). https://doi.org/10.1083/JCB.201812122 [50] D. Yang, X. Zhang, C. Wang, Y. Tang, J. Li, and J. Hu. Water-soluble multi-walled carbon nanotubes generated using Ce(IV)-induced redox radical polymerization. 991-996 in Progress in Natural Science, 19(8). https://doi.org/10.1016/j.pnsc.2008.10.010 [51] N. A. P. S. Buss, S. J. Henderson, M. McFarlane, J. M. Shenton, and L. de Haan (2012). The history and prospects of monoclonal antibody therapies. Current Opinion in Pharmacology, Vol. 12, No. 5, pp. 615-622. https://doi.org/10.1016/j.coph.2012.08.001 [52] Nanomaterials for cancer therapy: current status and future possibilities, Cheng, Z., Li, M., Dey, R., and Chen, Y. The Journal of Hematology and Oncology (Vol. 14, No. 1) published the findings. BioMed Central, Inc., doi:10.1186/s13045-02101096-0. [53] W. S. vanden Berg-Foels, A. A. Alexander-Bryant, and X. Wen (2013). Bioengineering strategies for developing personalized cancer treatments. Pages 1-59 in Advances in Cancer Research, Volume 118. https://doi.org/10.1016/B978-0-12407173-5.00002-9 The Academic Press, Inc. [54] Advantages and disadvantages of employing nanomaterials in the creation of tumor vaccines. Li, X. D., Gao, J. Y., Yang, Y., Fang, H. Y., Han, Y. J., Wang, X. M., and Ge, W. Pages 629-634 in OncoTargets and Therapy, Volume 6. https://doi.org/10.2147/OTT.S41902 [55] Liu, Z., Tabakman, S., Welsher, K., and Dai, H. Carbon nanotubes in biology and medicine: detection, imaging, and drug administration in vitro and in vivo. 85-120 in Nano Research, 2(2). https://doi.org/10.1007/s12274-009-9009-8 [56] A. Holland, P. Wick, A. Koehler, K. Schmid, and C. Som (2007). Examining the environmental and human health implications of carbon nanotubes. Environmental Health Perspectives, Vol. 115, No. 8, pp. 1125-1131. https://doi.org/10.1289/ehp.9652 [57] A. Szabó, C. Perri, A. Csató, G. Giordano, D. Vuono, and J. B. Nagy (2010). Carbon nanotube and related material synthesis technologies. 3092-3140 in Materials, 3(5). https://doi.org/10.3390/ma3053092 [58] Kumar, V., Sharma, N., and S. S. Maitra (2017). Nanoparticle toxicity testing in vitro and in vivo. International Nano Letters, vol. 7, no. 4, pp. 243-256. DOI: 10.1007/s40089-017-0221-3 [59] A. Kumar, K. Gupta, S. Dixit, K. Mishra, and S. Srivastava (2019). A discussion of the positive and negative effects of nanotechnology in agriculture. The International Journal of Environmental Science and Technology (Vol. 16, Issue 4, pp. 21752184) published the findings. Environmental and Energy Research and Studies Center. https://doi.org/10.1007/s13762-0182119-7
Copyright
Copyright © 2023 Promod Kumar B M, M M Aatifulla Baig, Shivarudrayya I Dharawadmath, Vikas C M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55787
Publish Date : 2023-09-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

